Enabling Faster, Safer Medication Access in the OR
Designing the next generation of operating room equipment to enable safer access to controlled substances.

Role
Lead Designer (Solo)
Project Manager
Product Owner
Tools
Figma
Adobe CC
Azure DevOps
Industry
Healthcare Technology
Overview
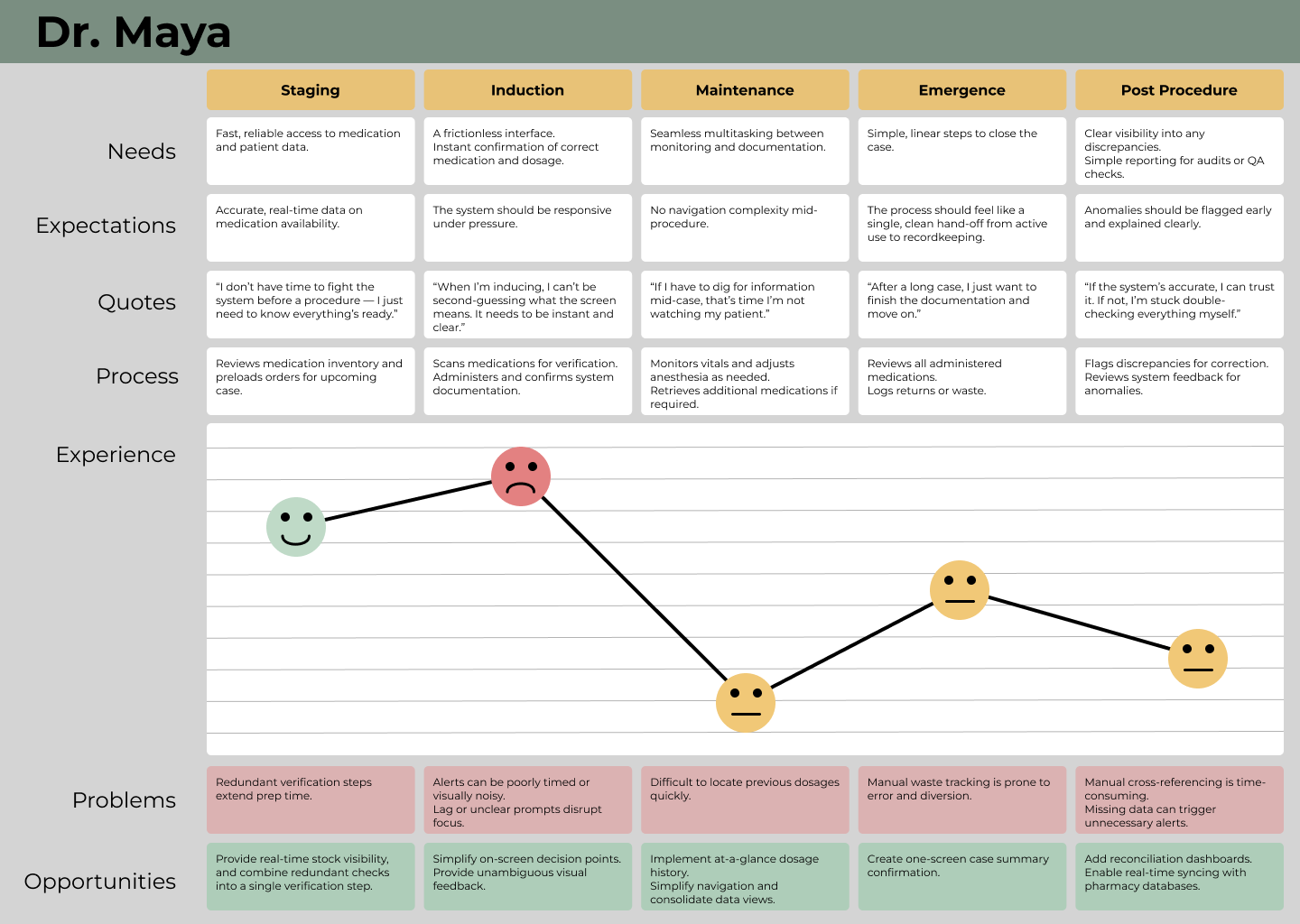
The Anesthesia Station was a strategically critical product that enabled our company’s first expansion into the operating room. For over a decade, we had been losing enterprise hospital deals because we lacked an OR-compatible solution while competitors offered full-suite medication management. Hospitals wanted a single-vendor partner, and without an anesthesia cart, we were routinely excluded from major purchasing decisions. This project represented our entry into a new clinical environment, with entirely new workflows, regulatory requirements, and technical constraints.
The stakes were unusually high. Failure meant losing one of the largest sales opportunities in company history, jeopardizing government funding, and putting a key partnership in the Canadian anesthesia market at risk. The timeline was aggressive, the budget was tightly constrained, and the team had no prior experience designing for the operating room. I served as the product owner and lead researcher, responsible for driving product direction across engineering, industrial design, regulatory strategy, and clinical research—balancing business risk, patient safety, and delivery under extreme uncertainty and pressure.
Discover
Entering an Unknown Clinical World
The OR was not just “another hospital workflow.”
The team initially assumed anesthesia workflows mirrored nursing and pharmacy workflows. Early interviews, including with the Chief of Anesthesia at Mayo Clinic, quickly disproved that.
The OR introduced new realities:
Extreme time pressure
Alert fatigue
Variable lighting
Sterility constraints
Posture differences
Deep integration with other hospital systems
This discovery forced a complete mental reset for the team.
Users, Tensions, and Competing Priorities
Speed vs. regulation was the core tension.
Anesthesiologists needed instant, frictionless access. Pharmacists needed strict compliance, auditing, and inventory control. Facilities varied widely in how they balanced speed versus security.
The key insight: configurability was not a feature — it was a requirement.
Physical, Technical, and Regulatory Constraints
Everything was constrained at once.
The cart had to fit through doorways and elevators, meet earthquake weight limits, survive aggressive sanitation, function in low light, and integrate with Epic. Technically, we hit early failures with sensor placement, RFID on liquids, lack of label standards, waterproof lighting, cable management, and serviceability.
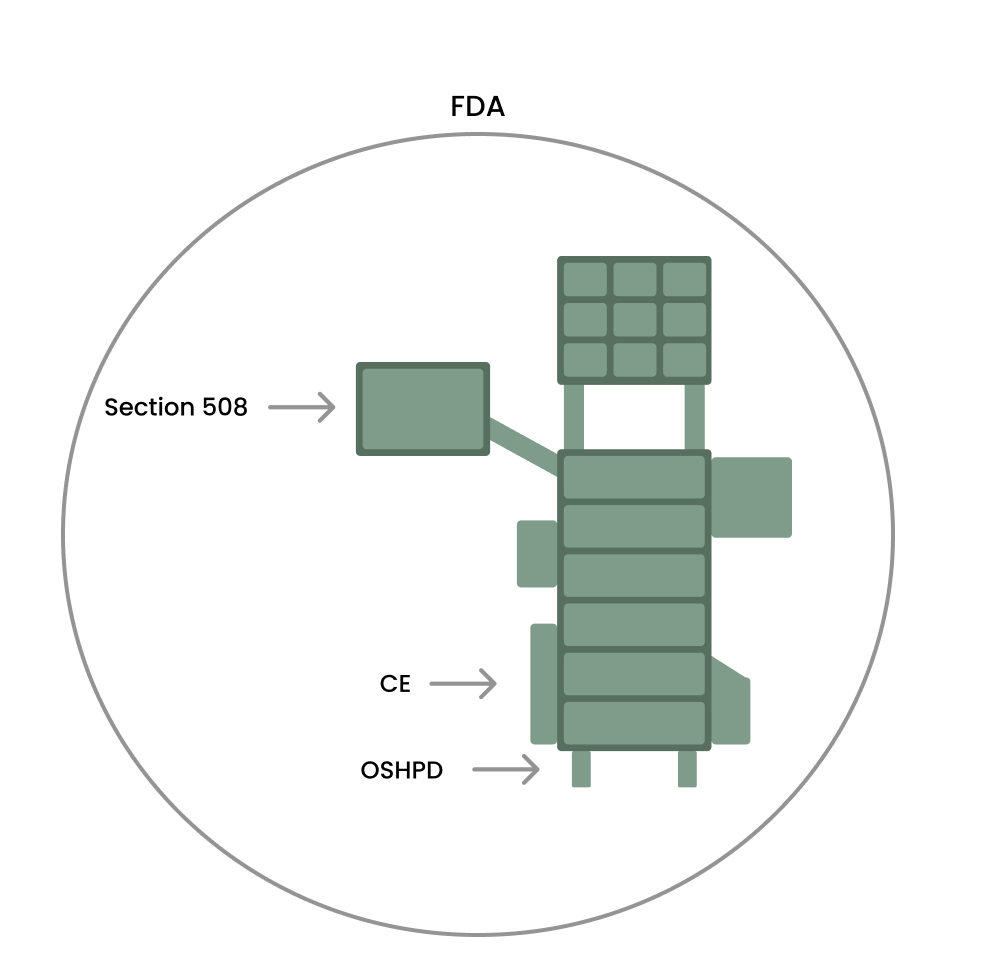
Regulatory discovery also reshaped the strategy — Class I FDA designation became the only viable path to hit the timeline.
Ownership, Scope Pressure, and Decision Authority
Discovery redefined my role.
I became the product owner, OR subject-matter authority, and cross-functional driver for the entire program. Scope pressure was constant, with repeated attempts to cut critical workflow requirements to protect timelines. I regularly had to defend user needs with real customer evidence, align leadership and engineering, and make high-impact decisions that directly shifted the product’s trajectory.
Define
Reframing the Real Problem
From “build a cart” to “enable safe, fast anesthesia workflows.”
Discovery made it clear this was not a hardware project alone. The real problem was designing a system that balanced speed of access, regulatory compliance, sterility, and inventory accuracy under extreme pressure. Facility needs varied, so a single rigid workflow would fail. The product had to be configurable by policy, not opinion.
Strategy: One Platform, Many Operating Rooms
Configurability became the core product strategy.
Instead of choosing between speed or security, we designed a system that allowed hospitals to tune access control, auditing depth, and verification steps by facility and jurisdiction. This prevented us from building a regional product and positioned the platform for North American scale.
Regulatory Path as a Product Decision
Class I FDA clearance unlocked the timeline.
We formally defined the regulatory approach as part of product strategy. By selecting Class I instead of Class II, we avoided clinical trials and lengthy approval cycles. This required precise scope control and carefully worded UI language to stay within regulatory boundaries while still delivering a usable product.
Scope Control and Non-Negotiables
Not everything made the cut. Some things could not be cut.
Time pressure forced real prioritization. Features like refrigerated medication storage were deferred despite their importance. Meanwhile, workflow-critical elements such as procedure-based medication loading, audit trails, and secure controlled substance handling remained non-negotiable. I owned those lines and defended them when scope pressure increased.
Develop
Speed of Access vs Security Was Not a Binary Choice
Early on, the team treated speed and security as a traditional tradeoff. Through direct hospital interviews, I uncovered that this balance varied by facility, region, and regulation. Instead of choosing one over the other, we built configurable access logic, allowing hospitals to define how aggressively security was enforced relative to speed.
This decision preserved safety while preventing usability failures in high-volume ORs.
Sensor Placement Nearly Broke the Workflow
Initial engineering assumptions placed sensors too far back in the drawers. During internal testing, one of our engineers surfaced that this caused delayed lid activation, drawer jamming, and excessive opening distance—dangerous in confined OR spaces.
I led validation testing to determine real-world activation thresholds. We moved sensors forward so lids triggered earlier, drawers opened less distance, and jams were eliminated. This single change prevented potential medication access failures during live procedures.
RFID Failure on Liquids Required a Parallel Tracking System
RFID struggled to read through liquid-filled vials, and the industry lacks a universal RFID label standard. We implemented a hybrid solution using custom labels (already used by some manufacturers) that allowed us to:
Track non-RFID vials
Support multiple RFID conventions
Maintain audit integrity for controlled substances
This ensured regulatory traceability even where modern infrastructure failed.
Sterility & Spill Protection in Pull-Out Trays
Pull-out trays introduced contamination risks from sticky substances and residue migration. We redesigned internal boundaries and tray interfaces to prevent fluid infiltration into protected storage zones while preserving ease of cleaning.
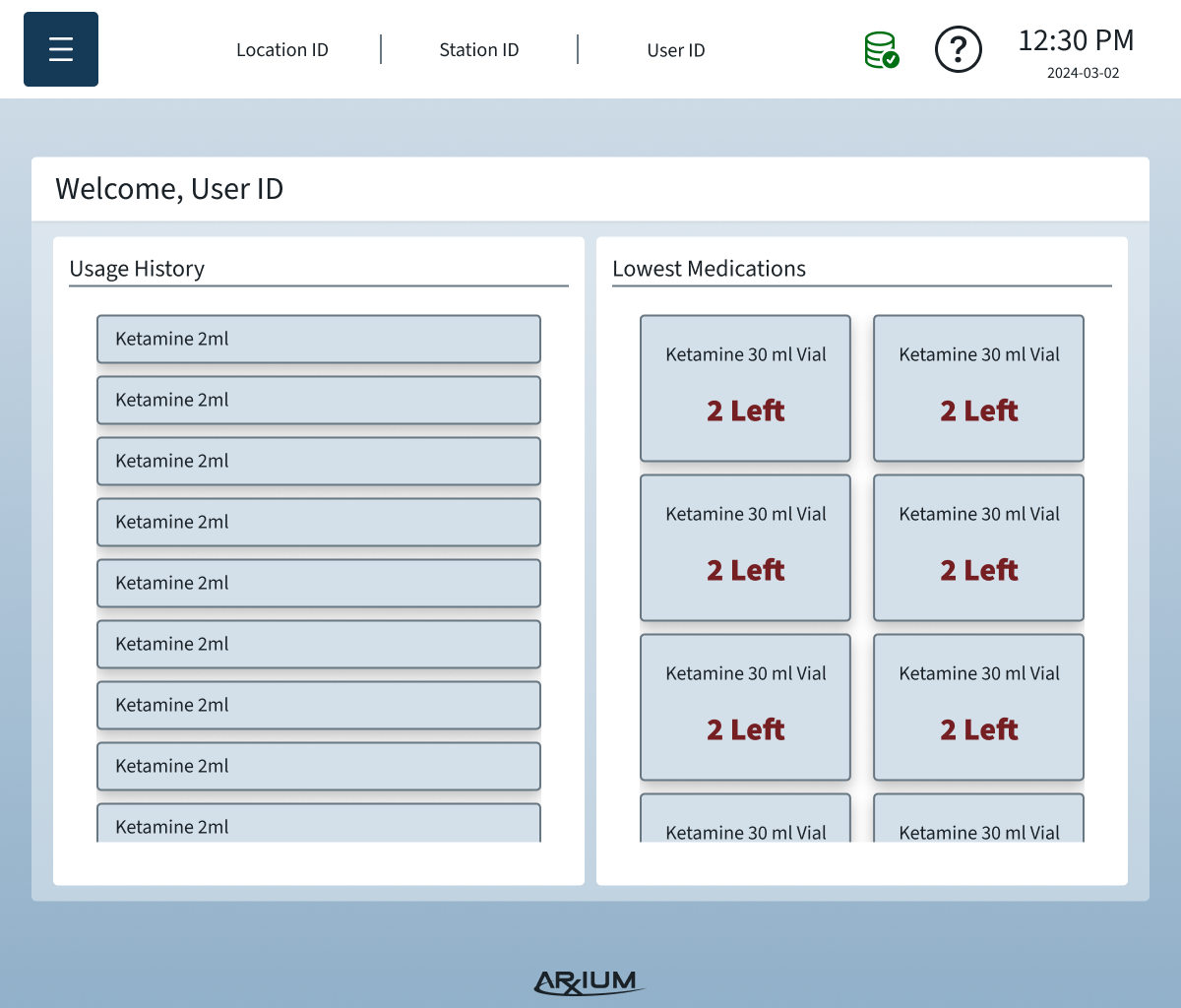
Information Architecture
The IA organized complex OR data and workflows into a clear, scannable structure that reduced errors and improved task completion speed:
Primary navigation by workflow: Users move through tasks in the order they occur during procedures — medication selection → scanning → logging → storage.
Contextual access: Only relevant information and options are visible depending on user role and current OR task.
Error-proof labeling & scanning: Clear states and prompts prevent accidental selection of wrong medications or compartments.
Support & troubleshooting: Persistent access to help content and alerts without disrupting critical workflows.
By designing the IA around real user needs and physical cart interactions, the system supported both novice and advanced users, minimized cognitive load, and allowed modular configurations without confusing the interface.
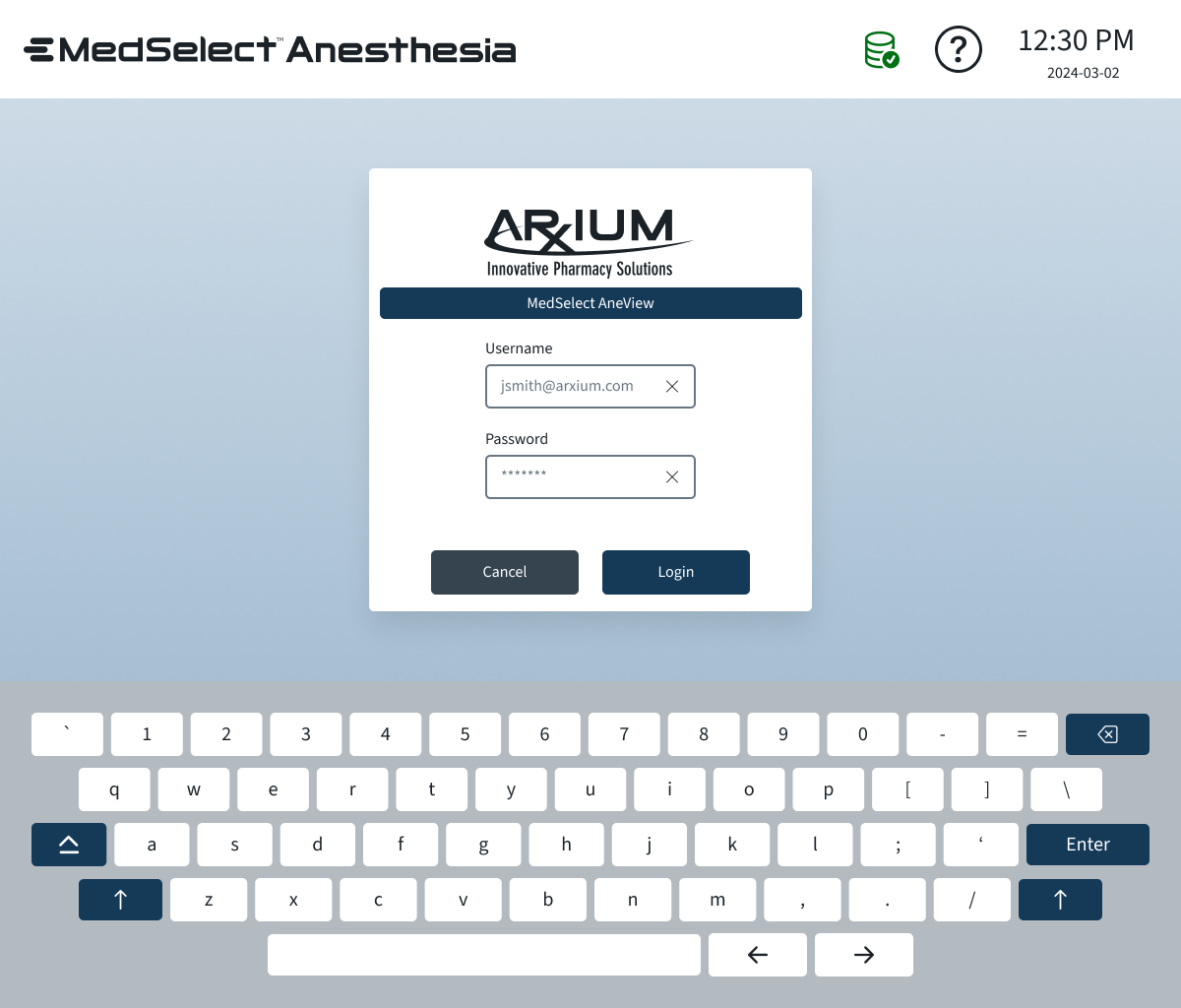
Wireframes
Wireframes were critical to iterate quickly on the UI before committing to hardware integrations. Low-fidelity sketches and digital wireframes allowed me to:
Map the user workflows: Show how anesthesiologists would select medications, scan labels, and access modular storage with minimal cognitive load.
Visualize modular storage: Demonstrate how trays and compartments could be reconfigured while keeping the UI consistent and intuitive.
Test feedback flows: Ensure lights, drawer sensors, and scanning confirmations were communicated clearly in the interface.
Optimize OR usability: Consider constraints like gloved hands, standing vs. sitting positions, high- and low-light environments.
Wireframes were iteratively refined after testing with engineering and early user feedback, ensuring that the UI reflected real-world OR workflows while remaining technically feasible.
Cognitive Load Under Pressure
OR users are advanced but overstimulated. Environment studies revealed notification fatigue and resistance to over-guided systems. We deliberately designed:
Minimal alerts
Fast confirmation states
Embedded scanning workflows for auditing
Error-proof controlled substance handling
This reduced mental load without reducing system control.
Deliver
Finalizing the UI for High-Stress, High-Stakes Use
I refined the anesthesia workflows to reduce cognitive load during procedures where seconds matter. This included tightening the medication-selection and labeling flows, simplifying scanning interactions, and ensuring everything aligned with OR-specific medication color conventions that the team initially overlooked. The goal was to make every step clear, fast, and predictable even when clinicians were operating under extreme time pressure.
Bridging Hardware and UI Into One Cohesive Experience
The UI had to synchronize seamlessly with tray lighting, sensor triggers, and the modular storage system. I worked closely with engineering as they developed a waterproof custom light, ensuring UI states clearly reflected hardware behavior. Because storage could be rearranged by facility, I updated UI logic so the system adapted to varied module configurations without confusing the user.
Validating the System in Simulated OR Scenarios
We ran OR-style simulations to see how the cart held up during fast-paced workflows—gloved users, rapid drawer openings, scanning in motion, and competing equipment. These sessions revealed friction points in scanning reliability, notification timing, and UI legibility. Each round of observation fed back into rapid iterations to ensure the system remained safe and intuitive in high-stress environments.
Driving a Collaborative, High-Transparency Handoff
Engineering was under intense timeline pressure, so I kept communication tight and transparent to minimize rework. I delivered precise UI states, error handling rules, and edge-case logic to ensure implementation matched clinical workflows. When scope cuts were proposed—such as removing the ability to add medications mid-procedure—I advocated for the user and preserved the features essential to safe practice.
Performing Design QA
During design QA, I validated that every UI interaction matched expectations and behaved correctly with the physical hardware: doors, trays, lights, and sensors. I negotiated necessary refinements, clarified ambiguous behaviors, and ensured the final build upheld both usability and safety requirements before sign-off.
Preparing for Launch and Real-World Adoption
Ahead of launch, I partnered with Sales, Support, and clinical champions to ensure the product could be introduced with minimal training overhead. I also prepared a prioritized list of next-iteration improvements—including the delayed refrigerated compartment—to ensure the roadmap reflected real-world needs discovered during testing.
Reflection
What This Project Proved
This project demonstrated my ability to lead in extreme uncertainty, where the problem, the market, and even the regulatory pathway were still forming in real time. I learned how to operate as a true product owner, setting direction when none existed, absorbing pressure from multiple sides, and making decisions that directly altered business outcomes.
It also reinforced that hardware, software, workflow, and regulation cannot be treated as separate disciplines in medical products. Every design choice triggered downstream effects in engineering, compliance, serviceability, and revenue. The ability to think in systems, not features, became the defining skill of this project.
What I Would Do Differently
With hindsight, I would formalize decision logs and risk registers earlier to reduce friction around scope and to create clearer historical accountability. I also would have pushed for earlier field prototyping in live OR environments to surface sterility, lighting, and posture conflicts sooner. Some late-stage adjustments could have been de-risked earlier with higher-fidelity pilots.
Organizational Maturity
Internally, this project established a reusable playbook for cross-functional, regulated product development. It clarified how product, engineering, regulatory, and leadership collaborate under real market pressure, setting the standard for future hardware and software programs.
Next Steps
Add the refrigerated medication compartment that was deferred due to timeline constraints
Introduce enhanced analytics for usage trends, waste reduction, and diversion monitoring
Expand modular storage configurations based on early user feedback
Improve workflow visibility and auditability for both anesthesiologists and pharmacists